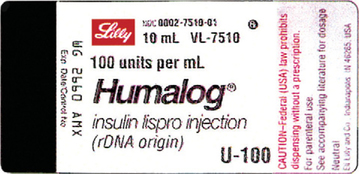
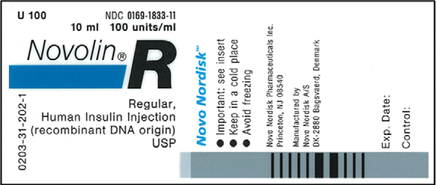
39 medication labels must include
Over the Counter (OTC) Drug Labels - Poison This is the easiest way to prevent errors and overdoses, because OTC medicines are often used without health professional advice. All over-the-counter (OTC) medication labels contain Drug Facts. Drug Facts include important information about the active ingredient (s), uses, warnings, doses, and directions. Serkalem Mekonnen, RN, BSN, MPH. The Over-the-Counter Medicine Label: Take a Look | FDA All nonprescription, over-the-counter (OTC) medicine labels have detailed usage and warning information so consumers can properly choose and use the products. Below is an example of what the OTC...
NCBOP - Pharmacist FAQs A. The following information must be on every prescription label: 1. Name and address of the dispensing pharmacy. 2. Serial number of the prescription. 3. Date of the prescription. 4. Name of the prescriber. 5. Name of the patient. 6. Name and strength of the drug. 7.

Medication labels must include
Understanding Drug Labels | Basicmedical Key The epinephrine injection label ( Fig. 5.6) indicates a dosage supply of 0.1 mg/mL, and the total volume of the ampul is 10 mL. Figure 5.4 The dosage strength of this dosage form of Diflucan ® (fluconazole) is 200 mg. Figure 5.5 The dosage strength of this drug is 125 mg (200,000 units) penicillin V in 5 mL. Chapter 8 NHA237 Flashcards | Quizlet If nothing is indicated on the prescription for refills, then the technician should: Check with the prescriber to see how many refills he or she wants. Tell the pharmacist. Enter the abbreviation, prn, (Latin, pro re nata) meaning as needed for refills. Enter zero refills. Enter zero refills. OTC Labeling Requirements - FindLaw The FDA conducted two studies to evaluate the effects these parameters have on the drug label readability, and after evaluating the studies concluded that a minimum type size of 6.7point is required to ensure that people over the age of 60 can read the label. Recognizing the space constraints, however, the FDA chose to allow type size as small ...
Medication labels must include. Prescriptions- Label Flashcards | Quizlet Here is a list of all the things that need to go on a prescription label for a non-control: 1. name of pharmacy 2. address of pharmacy 3. telephone number of pharmacy 4. number of the prescription 5. date the prescription is filled 6. name of patient**** 7. name of prescriber 8. initials of the pharmacist dispensing the Rx 4. Documenting Medications (MAR). | Aplmed Academy Prescription labels will contain: ... (OTC) medications include in the original manufacturer's bottle with the resident's name, OR Labeled by the pharmacy; ... If the licensed practitioner changes the dosage on prescribed medication, a new prescription must be filled by the pharmacy. The old medication cannot be administered and must be ... Drug labeling, Information about Drug labeling - FAQs Each product must contain a label with "Supplement Facts" in bold letters onthe front panel. This is the manufacturer's opportunity to identify the product. Below "Supplement Facts," the panel must state the serving size. This isdetermined by the manufacturer with no input from the FDA. Pharmacology Chapter 5 (Prescriptions and Labels) - Quizlet Every prescription must include the following:-DATE-Pysicians name, contact info, and DEA number-Patients name, address, and DOB-INSCRIPTION -SIGNATURE ... what other medications include medication labels. over-the-counter (OTC) drugs. OTC labels contain instructions on drug use based on _ and _ age
FDA's Labeling Resources for Human Prescription Drugs Human prescription drug labeling (1) contains a summary of the essential scientific information needed for the safe and effective use of the drug; and (2) includes the Prescribing Information,... Understanding the Critical Requirements for FDA Drug Labels What Should You Look For In FDA Drug Labels? Jump to Section — The FDA requires sturdy labels that must stay in position on the case and maintain ... Safe Labeling Helps Prevent OR Medication Errors Label information must include a medication's name and strength as well as amount when medications are mixed (as with antibiotic irrigations, tumescent and heparin solutions, and epinephrine). The unit of measure — percent, grams, milliliters, or units — must be recorded along with the date the medication is prepared. What's on a prescription label? - Knowledge is the best medicine In general your label will contain the following information:. Parts of a Prescription Label Rollover A-K below to see the various part of a prescription label. * A Drug Identification Number (DIN) is an eight digit number assigned by Health Canada to a drug product prior to being marketed in Canada.
PDF Labeling on the Sterile Field: Improve Patient Safety and Ensure Joint ... Labeling must include: Name of medication or solution, strength, date, and time Label one item at a time. Single items must also be labeled. Keep original containers of the medication or solution until the end of the procedure. Remember that containers and solutions brought in during the proce- What Is a Drug Label? | The Motley Fool Jun 27, 2016 at 11:27PM A drug label refers to all the printed information included with any dietary supplement, over-the-counter medicine, or prescription drug. They're strictly regulated by the... A Guide To Veterinary Prescription Label Requirements What Is Required On A Veterinary Prescription Label As shown in the above example, the actual container must include the following information: The name of the veterinary practice, its address, and contact information The veterinarian's name, the patient's name and species, and the client's last name How to Label a Medical Syringe Mar 14, 2022 — Unit dose medication packaging solutions including materials like labels for single-dose vials and syringes must also comply with federal ...
Labeling Information | Drug Products | FDA For more information on labeling, including Physician Labeling Rule (PLR) requirements, guidances, presentations, sample templates and format tools, and established pharmacologic class (EPC ...
Chapter 5: Prescriptions and Labels Flashcards | Quizlet Drug Labels Regulated by the Food and Drug Administration (FDA), which determines what needs to be on the label Dispensing pharmacist's label must include: Pharmacy name, address, and phone number Dispensing date Dispensing date may differ from the date on the prescription. Rx number, which identifies this unique prescription in the computer system
Ch 8 Pharmacy Flashcards & Practice Test - Quizlet A legal prescription label must include all of the following except: A. Directions for use B. Date the prescription was dispensed C. Name, address, and telephone number of the prescriber D. Name, address, and telephone number of the dispensing pharmacy Name, address, and telephone number of the prescriber
Patient Labeling 101 - Food and Drug Administration Patient labeling should be written at a 6 to 8 th grade reading level z Use of certain fonts: Verdana, Arial, or APHont size 11 or greater for better visibility z Use of text boxes, bold font, and...
What Information Should Be on Drug Labels? - MedicineNet Feb 3, 2022 — Labeling requirements for prescription drugs · Statement of identity · Brand name · Net quantity of contents · Statement of dosage.
How to Read Over-the-Counter and Prescription Drug Labels Some labels include a seventh section with a phone number to call if you have questions or comments. The Drug Facts label for the over-the-counter drug acetaminophen, known by the brand name Tylenol, includes information about ingredients, uses, warnings and directions. Active Ingredient and Purpose.
How to read prescription drug labels - BeMedwise Whenever you are prescribed a medication, you should read and follow the information in the medication's "label" in order to ensure your safety. All prescription medicine containers include information on the label including the patient's name, the name of the medicine, dosage and instructions on how often to take the medicine.
How Do I Use Prescription Drug Labeling | FDA Highlights of Prescribing Information. Section 1: Indications and Usage. Section 2: Dosage and Administration. Section 3: Dosage Forms and Strengths. Section 4: Contraindications. Section 5 ...
FDA Says Drug Labels Must Include Clear Guidance for Pregnant Wom Starting June 30, new drug labels will have categories for "Pregnancy," "Lactation," and "Females and Males of Reproductive Potential." "Pregnancy" will include information on ...
FDA Issues New Prescription Label Requirements Apr 20, 2022 — A human prescription drug label must include all of the legal information set forth by the FDA. Details on Abuse-Deterrent: The information that ...
Code of Federal Regulations Title 21 - CFR (1) The labeling must contain a summary of the essential scientific information needed for the safe and effective use of the drug. (2) The labeling must be ...
Medicines: packaging, labelling and patient information leaflets Labels must include warnings for safe use of the medicine. All products that contain paracetamol must include statutory warnings. Additional warning statements must be included on the packaging of...
Packaging and Labeling of Pharmaceutical Products Obtained ... by M Veronin · 2011 · Cited by 30 — In the United States, the legal requirements for a prescription label are set by federal law and state statutes. The container should be comparable to that ...
Pharmaceutical Labeling: Requirements & Guidelines To meet today's FDA regulations, labeling information on drugs must include the following in this order: - Product Name - Drug Facts Table - Active Ingredients - Purpose and Use - Warnings - Directions - Allergic Reactions - Inactive Ingredients
How to Label Prescription Medication for Veterinary Patients A label should include the following components: The name of the veterinary practice, its address, and contact information The veterinarian's name, the patient's name and species, and the client's...
OTC Labeling Requirements - FindLaw The FDA conducted two studies to evaluate the effects these parameters have on the drug label readability, and after evaluating the studies concluded that a minimum type size of 6.7point is required to ensure that people over the age of 60 can read the label. Recognizing the space constraints, however, the FDA chose to allow type size as small ...
Chapter 8 NHA237 Flashcards | Quizlet If nothing is indicated on the prescription for refills, then the technician should: Check with the prescriber to see how many refills he or she wants. Tell the pharmacist. Enter the abbreviation, prn, (Latin, pro re nata) meaning as needed for refills. Enter zero refills. Enter zero refills.










Post a Comment for "39 medication labels must include"