40 phase diagram with labels
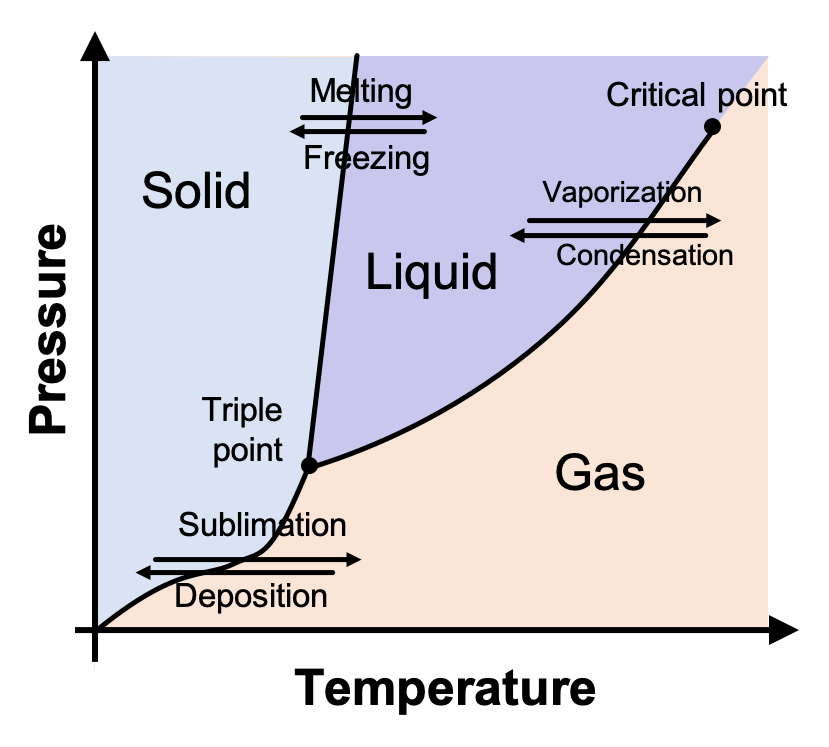
chem.libretexts.org › Phase_DiagramsPhase Diagrams - Chemistry LibreTexts Sep 10, 2022 · Phase diagram is a graphical representation of the physical states of a substance under different conditions of temperature and pressure. A typical phase diagram has pressure on the y-axis and temperature on the x-axis. As we cross the lines or curves on the phase diagram, a phase change occurs. Phase Diagrams - General College Chemistry - Brigham Young University The temperature and pressure conditions at which a substance exists in solid, liquid, and gaseous states are summarized in a phase diagram for that substance. Phase diagrams are combined plots of pressure-temperature equilibrium curves representing the relationships between phase transition temperatures and pressures. The point of intersection of any three curves in a phase diagram represents ...
Phase Diagram | Explanation, Definition, Summary & Facts A phase diagram is a graphical representation of the substance phases, consists of the curved lines and the space between the two lines represent a specific phase of the matter at given pressure and temperature, whereas any point at the curve lines shows the equilibrium between two phases. Phase diagram explanation
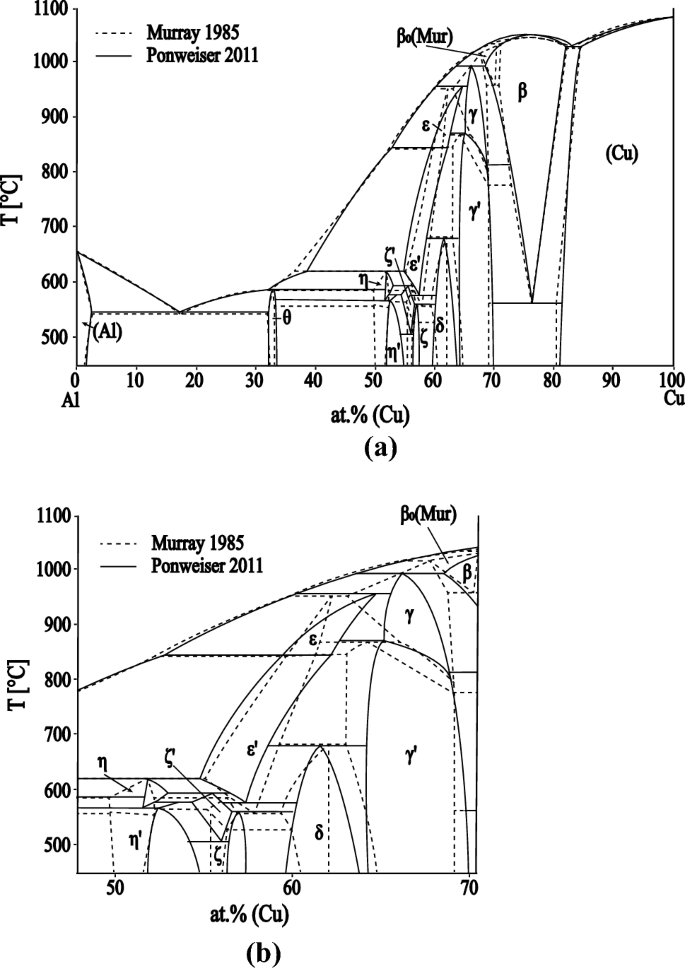
Phase diagram with labels
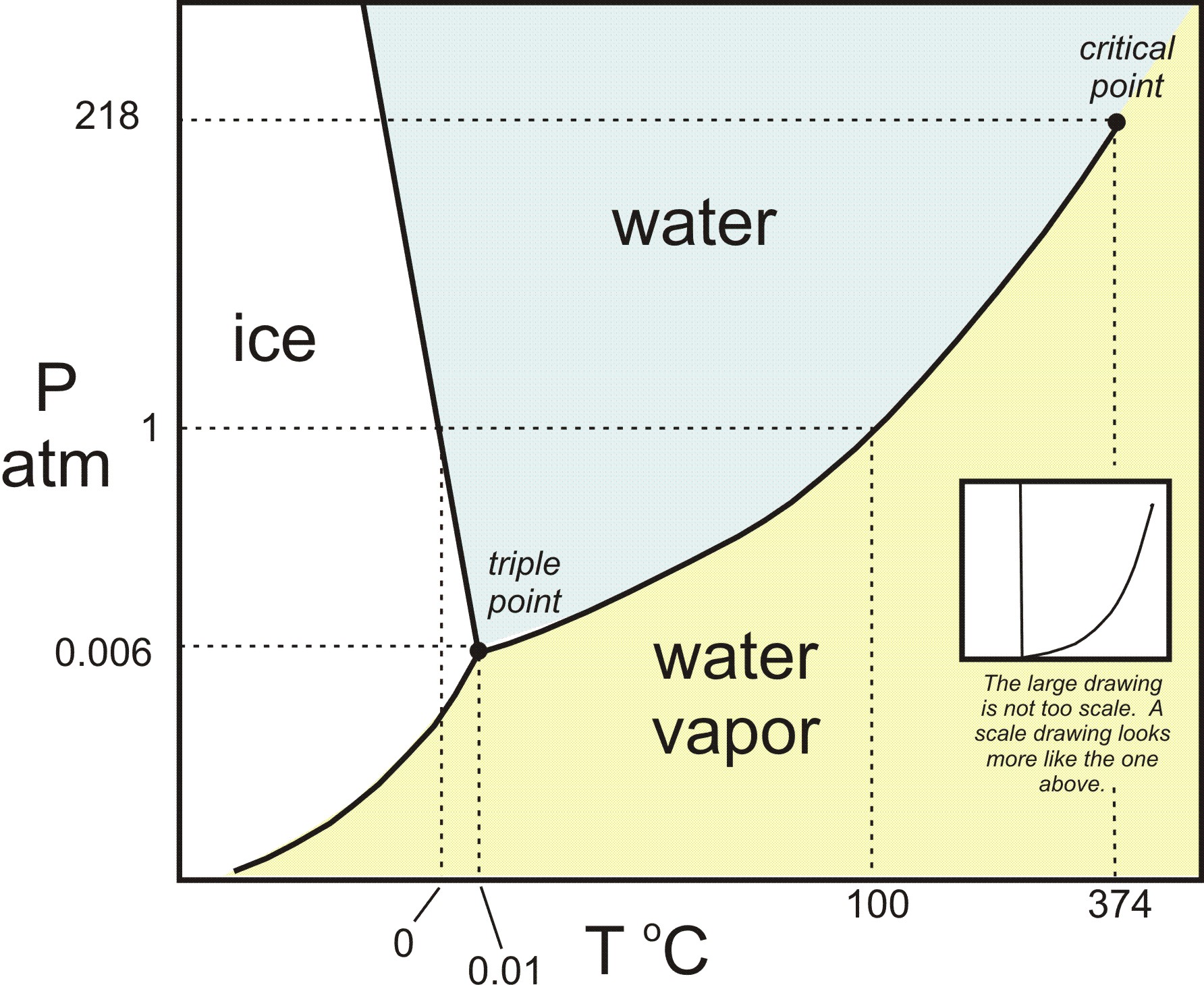
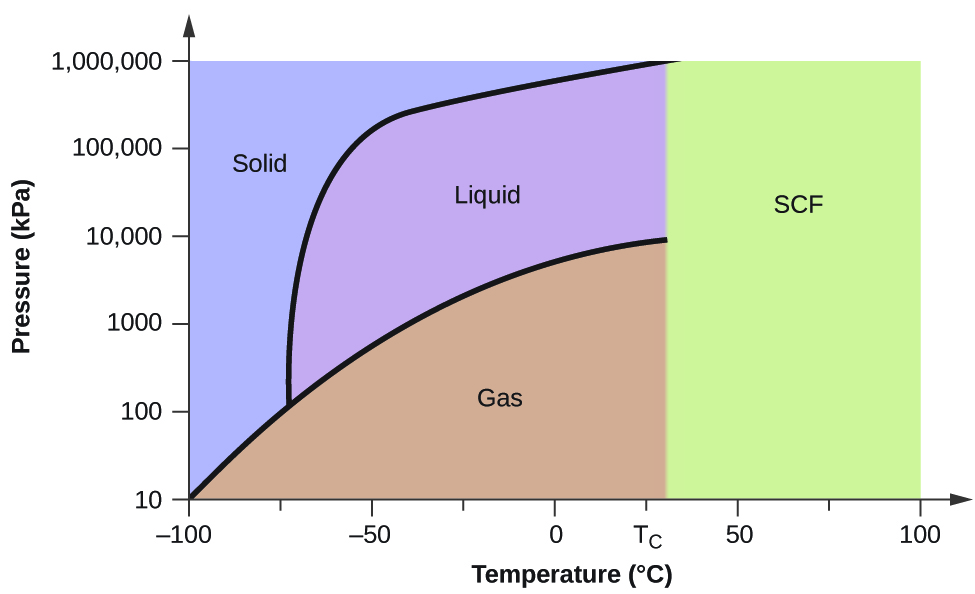
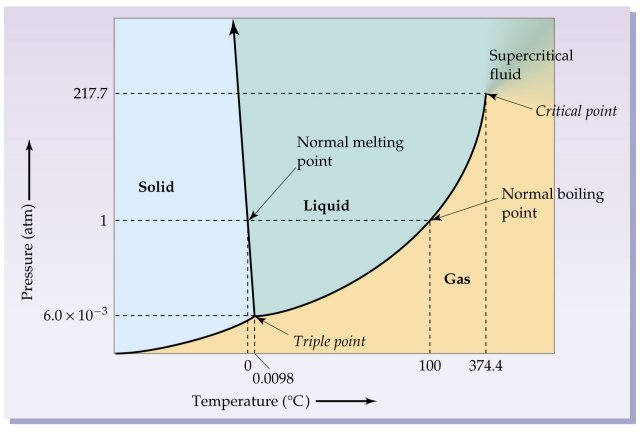
Phase Diagrams - Chemistry - University of Hawaiʻi We can use the phase diagram to identify the physical state of a sample of water under specified conditions of pressure and temperature. For example, a pressure of 50 kPa and a temperature of −10 °C correspond to the region of the diagram labeled "ice." Under these conditions, water exists only as a solid (ice). 10.4 Phase Diagrams - General Chemistry 1 & 2 We can use the phase diagram to identify the physical state of a sample of water under specified conditions of pressure and temperature. For example, a pressure of 50 kPa and a temperature of −10 °C correspond to the region of the diagram labeled "ice." Under these conditions, water exists only as a solid (ice). Phase diagram - Wikipedia The simplest phase diagrams are pressure-temperature diagrams of a single simple substance, such as water. The axes correspond to the pressure and temperature. The phase diagram shows, in pressure-temperature space, the lines of equilibrium or phase boundaries between the three phases of solid, liquid, and gas .
Phase diagram with labels. nces.ed.gov › nceskids › createagraphNCES Kids' Zone Test Your Knowledge - National Center for ... The NCES Kids' Zone provides information to help you learn about schools; decide on a college; find a public library; engage in several games, quizzes and skill building about math, probability, graphing, and mathematicians; and to learn many interesting facts about education. Phase Diagrams - Phases of Matter and Phase Transitions - ThoughtCo A phase diagram is a graphical representation of pressure and temperature of a material. Phase diagrams show the state of matter at a given pressure and temperature. They show the boundaries between phases and the processes that occur when the pressure and/or temperature is changed to cross these boundaries. Phase Diagram - Industrial Metallurgists The phase diagram indicates that an iron-carbon alloy with 0.5% carbon held at 900 °C will consist of austenite, and that the same alloy held at 650 °C will consist of ferrite and cementite. Furthermore, the diagram indicates that as an alloy with 0.78% carbon is slow cooled from 900 °C, it will transform to ferrite and cementite at about ... html.spec.whatwg.org › multipage › domHTML Standard The Document interface supports named properties.The supported property names of a Document object document at any moment consist of the following, in tree order according to the element that contributed them, ignoring later duplicates, and with values from id attributes coming before values from name attributes when the same element contributes both:
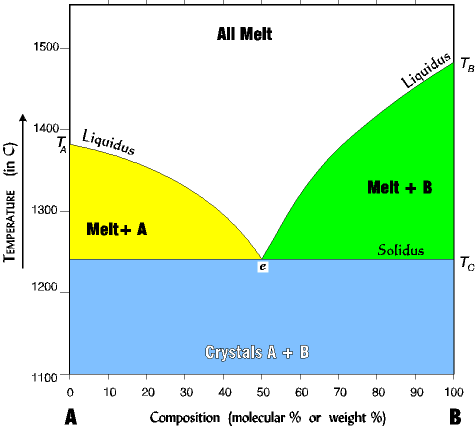
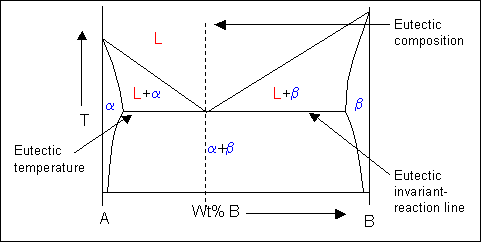
Phase Diagrams | Boundless Chemistry | | Course Hero A phase diagram is a graph which shows under what conditions of temperature and pressure distinct phases of matter occur. The simplest phase diagrams are of pure substances. These diagrams plot pressure on the y-axis and temperature on the x-axis. Although phases are conceptually simple, they are difficult to define precisely. PDF Phase Diagrams States of Matter and Phase Changes Terminology of Phase Diagrams Triple Point The triple point is the location on a phase diagram at which all three lines which divide the three states of matter meet. At this point, all three states of matter may exist at the same time. What is the pressure and temperature for the Phase diagrams - Big Chemical Encyclopedia 3 The phase diagram for the chemical A is shown below. Design a process to transform the gas at 300°C and 2 atm to a solid at 0°C and 1 atm without forming any liquid at any point in the process. Label the function of each unit and specify the temperature and pressure of each stream. Phase Diagram: Meaning and Types | Material Engineering This phase diagram consists of two points, two lines and three areas. The two points of the two pure metals A & B. The upper line, obtained by connecting the points showing the beginning of solidification is called liquidius line, and the lower line, determined by connecting the points showing the end of solidification is called the solidus line.
Label The Phase Changes Shown In The Diagram Below 27+ Pages ... On the phase diagram label the graphite phase. Freezing Condensation Sublimation Vaporization Deposition Melting Homework solution attached Purchase this answer to view it. Blog Archive Phase Diagrams Part 2 Diagram Material Science Calculus Label the diagram below for a summary of hormonal control of ovarian function during the luteal phase. › lifestyleLifestyle | Daily Life | News | The Sydney Morning Herald The latest Lifestyle | Daily Life news, tips, opinion and advice from The Sydney Morning Herald covering life and relationships, beauty, fashion, health & wellbeing Labeling Phase Change Diagrams | Chemistry | Study.com Steps for Labeling Phase Change Diagrams Step 1: Locate the triple point on the pressure vs. temperature phase diagram. This should look like the intersection of the letter Y . Step 2:... PDF Florida International University Florida International University
docs.swift.org › swift-book › LanguageGuideInitialization — The Swift Programming Language (Swift 5.7) The use of a two-phase initialization process makes initialization safe, while still giving complete flexibility to each class in a class hierarchy. Two-phase initialization prevents property values from being accessed before they’re initialized, and prevents property values from being set to a different value by another initializer unexpectedly.
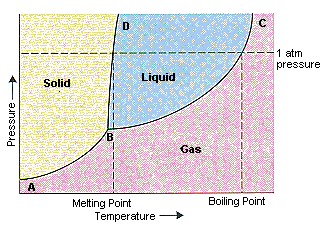
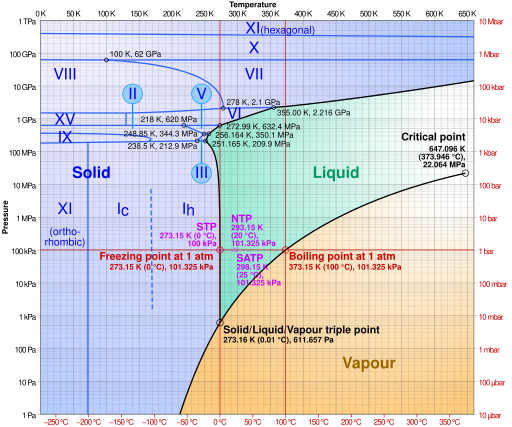
PDF 10. Phase diagrams Fig 10-1 P-T Phase diagram for H 2O To use such a diagram, we locate points in P-T space by specifying values for the two variables. If such a point lies in the area labeled "liquid," that would be the state (and phase) in which H 20 exists. If the point is on the line (e.g., DC), two phases would exist in equilibrium.
› createJoin LiveJournal Password requirements: 6 to 30 characters long; ASCII characters only (characters found on a standard US keyboard); must contain at least 4 different symbols;
What Is a Phase Diagram? - ThoughtCo A phase diagram is a chart showing the thermodynamic conditions of a substance at different pressures and temperatures. The regions around the lines show the phase of the substance and the lines show where the phases are in equilibrium. Parts of a Phase Diagram Typically, a phase diagram includes lines of equilibrium or phase boundaries.
› www › productsOriginlab GraphGallery Projected density of states (PDOS) for the H- ion located at fixed distances in front of a Na/Cu(111) surface Read more...
Phase Diagrams | General Chemistry - Lumen Learning We can use the phase diagram to identify the physical state of a sample of water under specified conditions of pressure and temperature. For example, a pressure of 50 kPa and a temperature of −10 °C correspond to the region of the diagram labeled "ice." Under these conditions, water exists only as a solid (ice).
Phase Diagrams - Highland The triple point of naphthalene is 80°C at 1000 Pa. Use these data to construct a phase diagram for naphthalene and label all the regions of your diagram. Argon is an inert gas used in welding. It has normal boiling and freezing points of 87.3 K and 83.8 K, respectively. The triple point of argon is 83.8 K at 0.68 atm. Use these data to ...
How to Label a Phase Diagram | Chemistry | Study.com Steps for Labeling a Phase Diagram Step 1: Distinguish between the different states of matter at a given temperature and pressure using a phase diagram. Step 2: Use the provided vocabulary...
Phase Diagrams | Chemistry for Majors - Lumen Learning We can use the phase diagram to identify the physical state of a sample of water under specified conditions of pressure and temperature. For example, a pressure of 50 kPa and a temperature of −10 °C correspond to the region of the diagram labeled "ice." Under these conditions, water exists only as a solid (ice).
15 3c Labeling a typical simple phase diagram - YouTube About Press Copyright Contact us Creators Advertise Developers Terms Privacy Policy & Safety How YouTube works Test new features Press Copyright Contact us Creators ...
Phase Diagram Labels - 18 images - label the phase diagram of pure ... Phase Diagram Labels. Here are a number of highest rated Phase Diagram Labels pictures on internet. We identified it from honorable source. Its submitted by dealing out in the best field. We allow this kind of Phase Diagram Labels graphic could possibly be the most trending subject later we allowance it in google help or facebook.
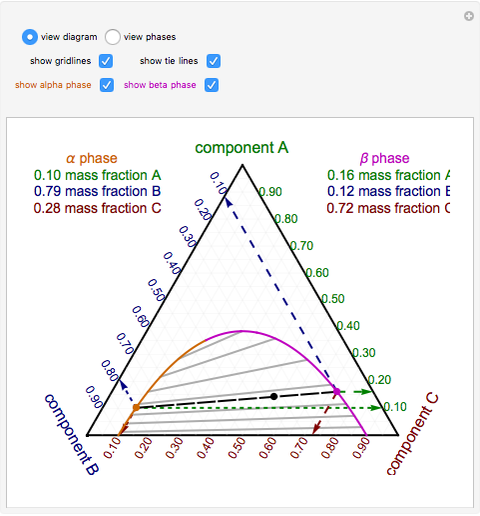
How to Determine Phase Diagram? (4 Methods) | Metallurgy The followings are some of the methods used in the determination of the phase diagrams: 1. Thermal analysis 2. Dilatometry 3. Microscopic methods ADVERTISEMENTS: 4. X-ray diffraction methods 5. Electrical-resistivity methods Method # 1. Thermal Analysis:
How to label a blank phase diagram - YouTube Phase diagrams are a super helpful resource for materials scientists. Labeling them can be challenging, but, fortunately, there are some simple rules to follow. The top portion will be liquid,...
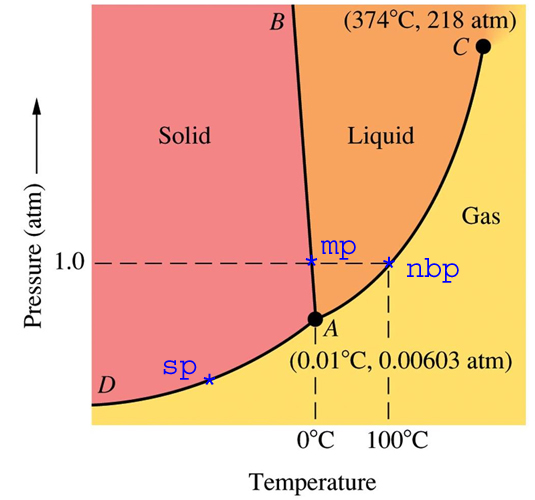
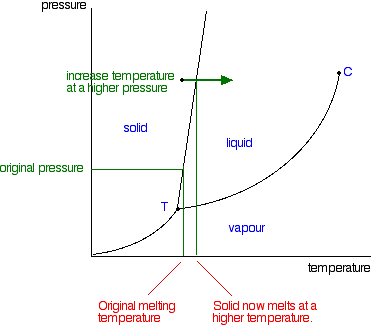
Phase Diagrams - Purdue University The figure below shows what happens when we draw a horizontal line across a phase diagram at a pressure of exactly 1 atm. This line crosses the line between points B and D at the melting point of the substance because solids normally melt at the temperature at which the solid and liquid are in equilibrium at 1 atm pressure.
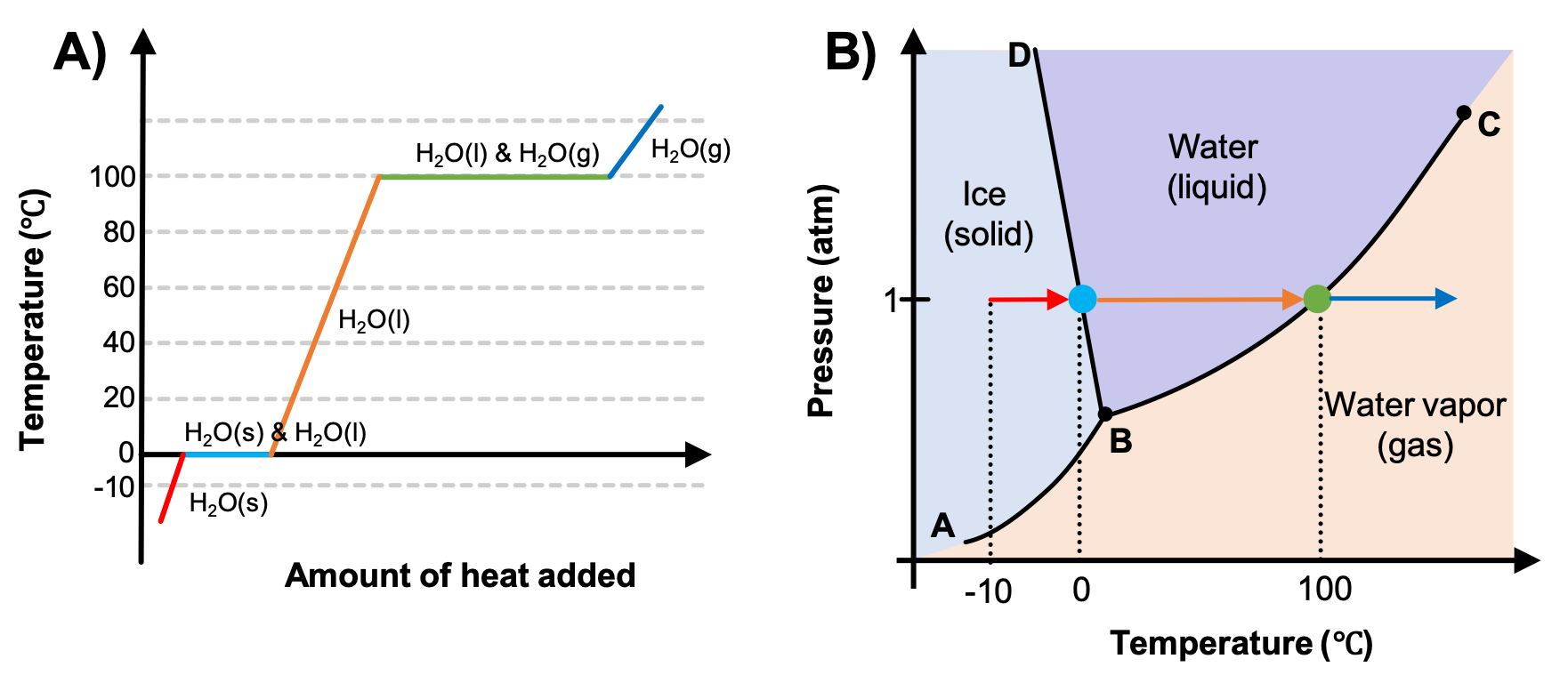
Phase Diagram of Water - Explanation and Diagrammatic ... - BYJUS A phase diagram is a graphical representation of the various phases of a substance or mixture of substances that coexist in thermodynamic equilibrium, and undergo phase changes under different working conditions, such as temperature, pressure, or volume. The water system is divided into three phases: ICE (S), WATER (L), and WATER VAPOUR (G)
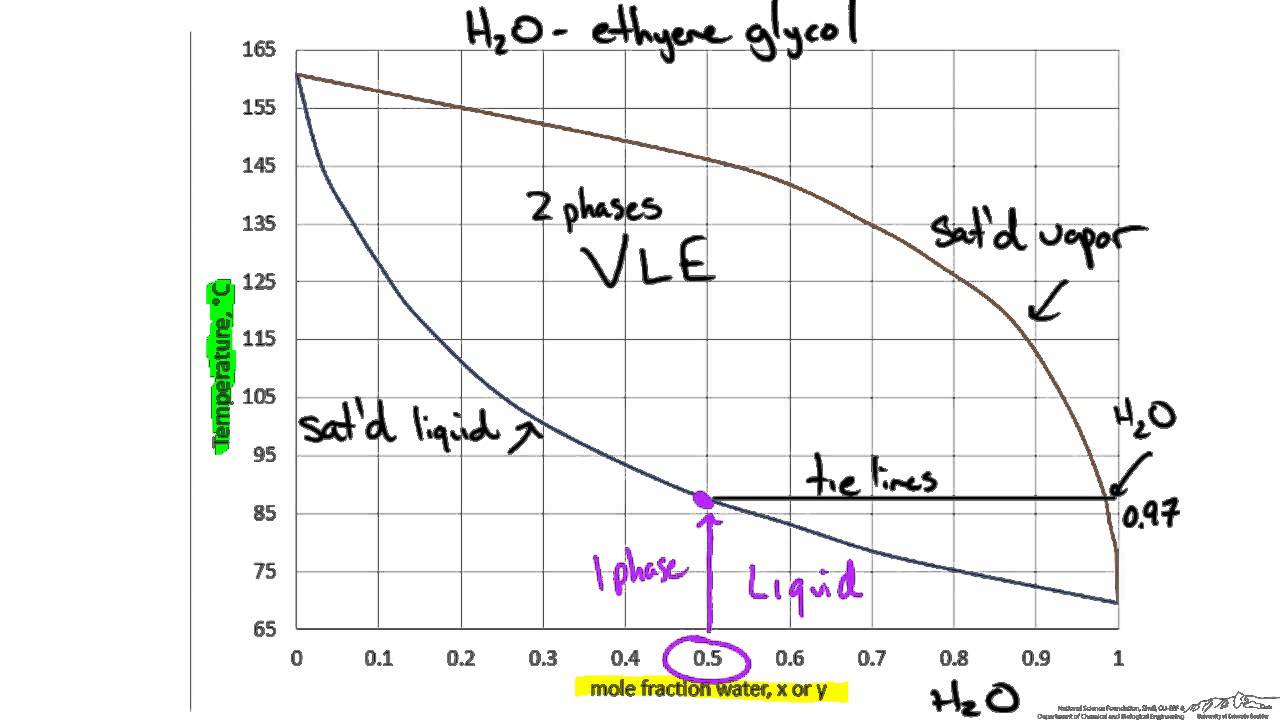
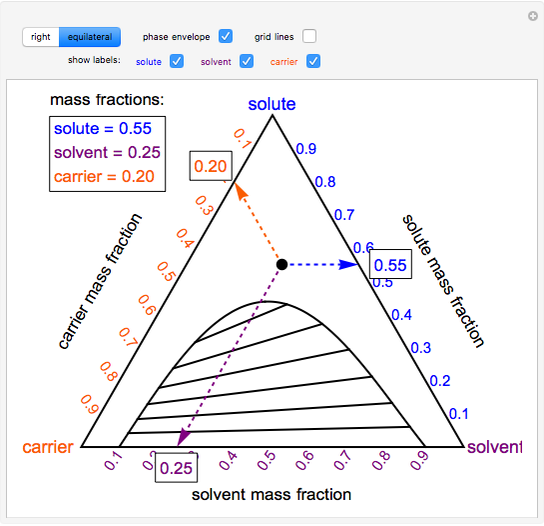
PDF Interpreting Phase Diagrams - University of Houston In the field labeled Solid A and liquid, a two phase tie line intersects the Solid A side of the diagram and the liquidus. Note that each two phase tie line always intersects pure A but a liquid with a different bulk composition. The field of Solid B + liquid behaves in a similar fashion. The 4th field is labeled Solid A plus Solid B.
Phase Diagrams | Chemistry | | Course Hero We can use the phase diagram to identify the physical state of a sample of water under specified conditions of pressure and temperature. For example, a pressure of 50 kPa and a temperature of −10 °C correspond to the region of the diagram labeled "ice." Under these conditions, water exists only as a solid (ice).
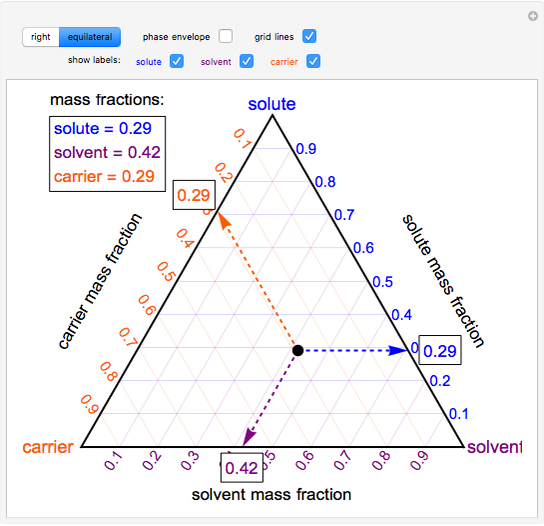
7+ label the phase diagram of pure solvent and a solution Phase Diagram Labels. The phase diagrams for the pure solvent solid lines and the solution non-volatile solute dashed line are recorded below. Identify the normal freezing fpsolv and boiling bpsolv points for the pure solvent and the normal. The ratio of the moles of solute in solution to the total number of moles.
Phase diagram - Wikipedia The simplest phase diagrams are pressure-temperature diagrams of a single simple substance, such as water. The axes correspond to the pressure and temperature. The phase diagram shows, in pressure-temperature space, the lines of equilibrium or phase boundaries between the three phases of solid, liquid, and gas .
10.4 Phase Diagrams - General Chemistry 1 & 2 We can use the phase diagram to identify the physical state of a sample of water under specified conditions of pressure and temperature. For example, a pressure of 50 kPa and a temperature of −10 °C correspond to the region of the diagram labeled "ice." Under these conditions, water exists only as a solid (ice).
Phase Diagrams - Chemistry - University of Hawaiʻi We can use the phase diagram to identify the physical state of a sample of water under specified conditions of pressure and temperature. For example, a pressure of 50 kPa and a temperature of −10 °C correspond to the region of the diagram labeled "ice." Under these conditions, water exists only as a solid (ice).



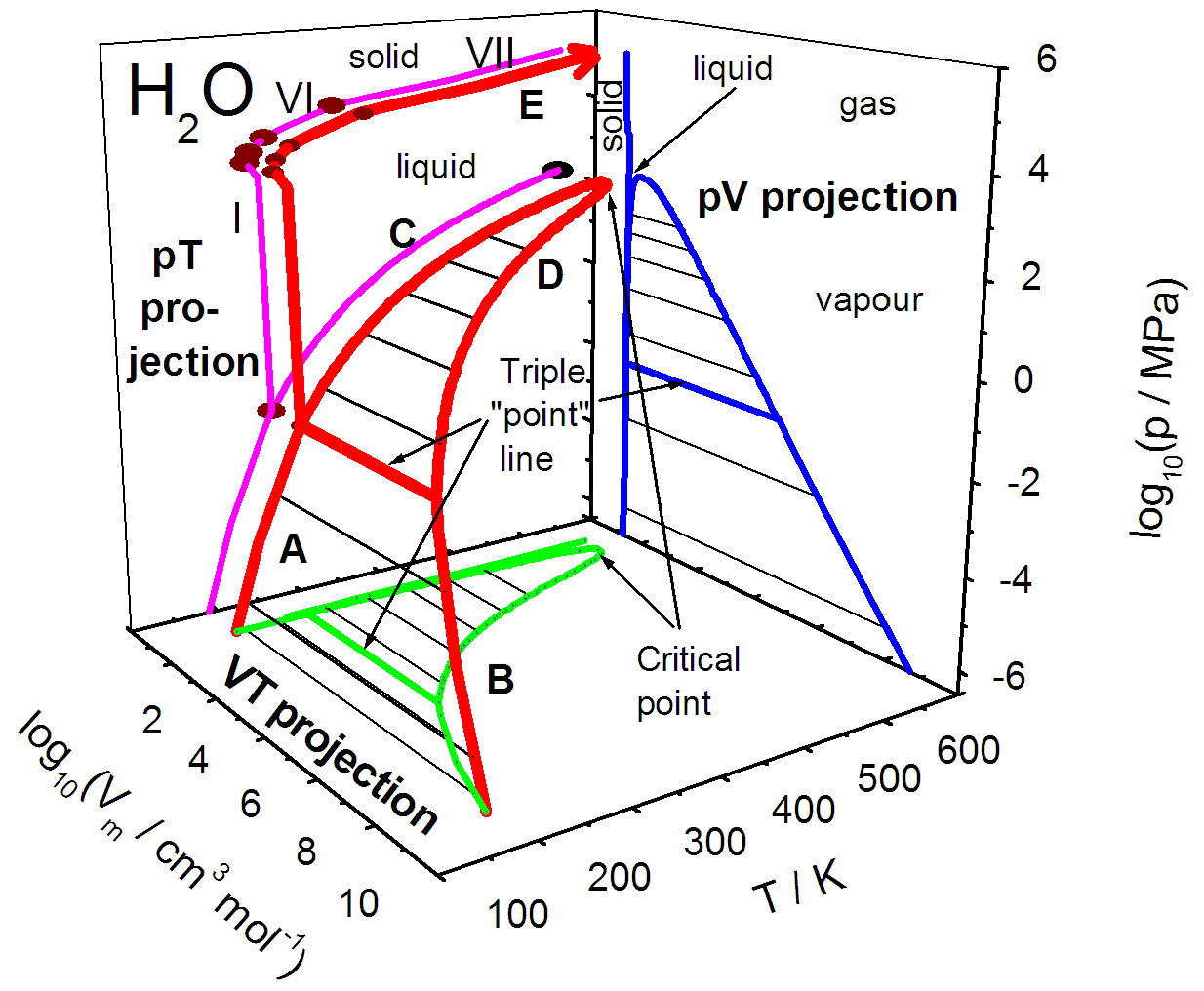
















Post a Comment for "40 phase diagram with labels"